Background
Hyperthyroidism refers to overactivity of the thyroid gland, which leads to excessive release of thyroid hormones and consequently accelerated metabolism in the peripheral tissues. Thyrotoxicosis refers to the clinical effects of unbound thyroid hormones, whether or not the thyroid gland is the primary source. Hyperthyroidism is the most prevalent cause of thyrotoxicosis but a relatively rare condition in children. Graves disease, a form of thyroid autoimmune disorder featuring hyperthyroidism, [1] in childhood accounts only for 1-5% of all patients with Graves disease but is the cause of more than 95% of cases of pediatric thyrotoxicosis. [2]
In children and adolescents, the symptoms of Graves disease, such as hyperthyroidism, may appear insidiously over months. Early diagnosis requires a high degree of suspicion. The main clinical features are goiter, tachycardia, nervousness, hypertension, tremor, weight loss despite increased appetite, hyperactivity, irregular menses, ophthalmopathy, heat intolerance, and diarrhea.
The Japanese Thyroid Association (JTA) includes the following diagnostic criteria for childhood-onset Graves disease [3] :
Clinical findings
-
Signs of thyrotoxicosis such as tachycardia, weight loss, finger tremor, and sweating.
-
Diffuse enlargement of the thyroid gland.
-
Exophthalmos and/or specific ophthalmopathy
Laboratory findings
-
Elevation in serum free thyroxine (FT4) and/or free triiodothyronine (FT3) level
-
Suppression of serum thyroid stimulating hormone (TSH): less than 0.1 μU/mL
-
Positive for anti-TSH receptor antibody (TRAb or TSH binding inhibitory immunoglobulin [TBII]) or thyroid stimulating antibody (TSAb)
-
Elevated radioactive iodine (or 99mTcO4–) uptake to the thyroid gland
Graves disease is diagnosed with at least one of the clinical findings and all four laboratory findings. Graves disease is suspected with at least one of the clinical findings and the first three laboratory findings or at least one of the clinical findings and first two laboratory findings. Elevation in serum FT4 has usually been present for at least 3 months.
Treatment approaches include antithyroid drugs (ATDs), which are commonly used as the first-line treatment, or thyroid ablation with either radioactive iodine therapy or thyroidectomy. However, ATDs have a high rate of relapse. Treatment with radioiodine or surgical subtotal thyroidectomy is very effective, but most patients develop hypothyroidism and require lifelong thyroid replacement. Although remission in adults is 40-60%, only about one third of children achieve remission. [2]
See also Pediatric Graves Disease; Hyperthyroidism and Thyrotoxicosis; Hyperthyroidism, Thyroid Storm, and Graves Disease in Emergency Medicine; Pediatric Hypothyroidism; Graves Disease; Orbital Decompression for Graves Disease; and Thyroid-Associated Orbitopathy.
Pathophysiology
Understanding the normal physiology of the thyroid gland is necessary to understand the pathophysiology of hyperthyroidism. Secretion of thyroid hormone is controlled by the interaction of stimulatory and inhibitory factors. The thyroid, like other endocrine glands, is controlled by a complex feedback mechanism (see the image below).
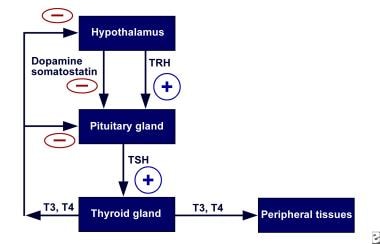 Pediatric Hyperthyroidism. Schematic representation of the negative/positive feedback system with respect to the hypothalamic-pituitary-thyroid axis. TRH = thyrotropin-releasing hormone; TSH = thyroid-stimulating hormone.
Pediatric Hyperthyroidism. Schematic representation of the negative/positive feedback system with respect to the hypothalamic-pituitary-thyroid axis. TRH = thyrotropin-releasing hormone; TSH = thyroid-stimulating hormone.
The release of thyrotropin, or thyroid-stimulating hormone (TSH), from the anterior pituitary gland is stimulated by low circulating levels of thyroid hormones (negative feedback) and is under the influence of thyrotropin-releasing hormone (TRH), somatostatin, or dopamine. Thyrotropin then binds to TSH receptors on the thyroid gland, setting off a cascade of events within the thyroid gland, leading to the release of the thyroid hormones, primarily thyroxine (T4) and, to a lesser degree, triiodothyronine (T3). Elevated levels of these hormones, in turn, act on the hypothalamus and anterior pituitary gland, decreasing synthesis of TSH. Under physiologic conditions, the levels of circulating free thyroid hormones are tightly regulated.
The TSH receptor belongs to one of the families of proteins known as G-protein–coupled receptors. The TSH receptor is a large protein embedded in the cell membrane. It contains an extracellular domain (that binds) TSH and an intracellular domain that acts via a G-protein second messenger system to activate thyroid adenyl cyclase, yielding cyclic adenosine monophosphate (cAMP). Effects of TSH are largely mediated through this second messenger system.
Synthesis of thyroid hormone depends on an adequate supply of iodine. Dietary inorganic iodide is transported into the gland by an iodide transporter (iodide pump on the surface of thyroid follicular cells). Iodide is then converted to iodine and bound to tyrosine residues on thyroglobulin by the enzyme thyroid peroxidase via a process called organification. The result is the formation of monoiodotyrosine (MIT) and diiodotyrosine (DIT). Coupling of MIT and DIT results in the formation of T3 and T4, which are then stored with thyroglobulin in the extracellular colloid of the thyroid’s follicular lumen. The thyroid contains a large supply of its preformed hormones.
To release thyroid hormones, thyroglobulin is first endocytosed into the follicular cell and then degraded by lysosomal enzymes. Stored T4, and T3 to a lesser degree, then diffuse into the peripheral circulation. Most T4 and T3 in the peripheral circulation are bound to plasma proteins and are inactive. Only 0.02% of T4 and 0.3% of T3 molecules are free (unbound to other proteins). T4 can be monodeiodinated to form either T3 or reverse T3 (rT3), but only free T3 is metabolically active. T3 acts by binding to nuclear receptors (DNA-binding proteins in cell nuclei), regulating the transcription of various cellular proteins.
Any process that causes an increase in the peripheral circulation of unbound thyroid hormone can cause signs and symptoms of hyperthyroidism. Disturbances of the normal homeostatic mechanism can occur at the level of the pituitary gland, the thyroid gland, or in the periphery. Regardless of etiology (see Etiology), the result is an increase in transcription in cellular proteins causing an increase in the basal metabolic rate. In many ways, signs and symptoms of hyperthyroidism resemble a state of catecholamine excess, and adrenergic blockade can improve these symptoms.
Etiology
Childhood Graves disease
Graves disease accounts for the majority of cases of hyperthyroidism in children and adolescents. It is an autoimmune disease associated with thyroid-stimulating hormone (TSH) receptor-stimulating antibodies. Classic Graves disease includes the triad of hyperthyroidism, ophthalmopathy, and dermopathy. Dermopathy is characterized by localized myxedema and is extremely unusual in children.
Hyperthyroidism in Graves disease is caused by thyroid-stimulating immunoglobulins (TSIs) of the immunoglobulin G1 (IgG1) subclass. These antibodies bind to the extracellular domain of the thyroid-stimulating hormone (TSH) receptor and activate it, causing follicular growth and activation and release of thyroid hormones. Patients may have a number of other antithyroid antibodies, some of which are also thyroid receptor antibodies (TRAbs) but which may not activate the receptor. Interplay between these various antibodies likely determines the course and severity of disease.
Initial stimulus for the formation of TSI is not known. Some microorganisms, such as Yersinia species, have proteins that bind TSH. Infection with these organisms possibly induces antibodies that cross-react with the TSH receptor. Some clinical evidence supports this hypothesis. Other evidence suggests that viral infection of the thyroid may be involved. Viruses may induce expression of major histocompatibility (MHC) class II antigens on the surface of thyroid follicular cells, leading to an immune response and autoantibody formation.
Ophthalmopathy of Graves disease is multifactorial. Some symptoms, such as lid lag and lid retraction, are caused by sympathomimetic effects of the thyrotoxicosis and resolve when the patient becomes euthyroid. Other symptoms may be a result of an autoimmune reaction against the muscles or fibroblasts of the orbit. These symptoms may not resolve with correction of the thyroid dysfunction. Theoretically, a shared antigen or antigens between the thyroid gland and the contents of the orbit may be present.
Neonatal Graves disease
Graves disease in neonates accounts for fewer than 1% of all cases of hyperthyroidism in pediatric patients. The frequency of neonatal Graves disease is equal in males and females, reflecting the underlying pathophysiology. Pathogenesis and course of the disorder in this age group are unique. Virtually all patients have a maternal history of Graves disease, either during the pregnancy or at some time in the past.
Neonatal Graves disease is caused by the transplacental passage of TSI. The mother may have clinical hyperthyroidism, may be on antithyroid medication, or may have a history of radioablation therapy or thyroid surgery. Rarely, the mother has a history of chronic lymphocytic (Hashimoto) thyroiditis. Maternal elevation of TSI titers is a consistent finding in these cases.
Neonatal Graves disease is rare even among mothers with known hyperthyroidism. Only 1 in 70 infants of thyrotoxic mothers has clinical symptoms. A maternal TSI level must be very high (>5 times normal) to produce clinical disease in the neonate.
Because neonatal Graves disease is caused by maternal IgG antibodies, it is self-limited and resolves when the child is aged 3-4 months. Symptoms of hyperthyroidism rarely persist longer. More persistent hyperthyroidism in neonates is likely to reflect a different pathogenesis, such as an activating mutation of the TSH receptor.
Prenatally, the thyroid gland is fully responsive at 28 weeks' gestation. These babies can be treated with methimazole, which is given to the mother. Methimazole may be preferable to propylthiouracil (PTU), because methimazole binds less to plasma proteins and therefore crosses the placenta more easily. However, the risk of cutis aplasia may be increased in infants born to mothers who have taken methimazole during pregnancy. PTU is associated with a higher incidence of idiopathic liver failure.
If the mother is taking antithyroid drugs, infants are usually born asymptomatic. Signs and symptoms may become manifest when antithyroid medications that have crossed the placenta are cleared from the infant's bloodstream. Signs are similar to those in older children with thyrotoxicosis, such as tachycardia, wide pulse pressure, irritability, tremor, and hyperphagia with poor weight gain. The baby may have exophthalmos and goiter.
Neonates have a much higher risk of morbidity and mortality from cardiac disease. In severe cases, congestive heart failure (CHF) can be observed. In addition, the goiter can occasionally be large enough to cause airway compression.
Long-term effects can include craniosynostosis and developmental delay. This latter finding occurs even in the face of early diagnosis and treatment, which suggests that prenatal exposure to high levels of thyroid hormone may have early effects that cannot be overcome after birth.
Toxic adenomas, toxic nodular goiter, carcinomas
Isolated toxic adenoma (Plummer disease) and toxic nodular goiter of adulthood are rare in children. Although follicular or papillary carcinomas can appear as a thyroid mass, they are virtually always nonfunctioning and therefore rarely cause hyperthyroidism.
McCune-Albright syndrome
Hyperthyroidism associated with McCune-Albright syndrome is rare. This syndrome includes polyostotic fibrous dysplasia, café-au-lait spots, or other endocrinopathies. The most common endocrinopathy is precocious puberty, but hyperthyroidism also can be observed.
In addition to other signs and symptoms of hyperthyroidism, patients initially present with a diffuse goiter. The goiter may become a multinodular goiter over time.
Hyperthyroidism in McCune-Albright syndrome is due to a mutation in the α subunit of the G regulatory protein that links the TSH receptor with adenylate cyclase. This mutation results in constitutive activation of the protein and production of cAMP, bypassing the normal requirement for TSH activation of the receptor. Biopsies revealed some tissue with the normal protein and other tissue with the mutated protein, suggesting that the mutation may occur postfertilization and providing an explanation for its heterogeneous expression.
Unlike the hyperthyroidism of Graves disease, McCune-Albright syndrome does not spontaneously remit. Treatment with antithyroid medications provides only temporary benefit. Therefore, the treatment of choice is surgical resection (thyroidectomy) or radioactive iodine ablation. Injection of ethanol into toxic nodules under ultrasonographic guidance has been used with some success in adults. [4]
Subacute thyroiditis
Subacute thyroiditis is generally associated with a viral upper respiratory infection. Signs and symptoms of hyperthyroidism are mild and generally overshadowed by fever and thyroid tenderness. The area surrounding the thyroid may be erythematous and warm, and the gland is always tender to touch.
Hyperthyroidism in these patients is caused by inflammation of the thyroid gland and subsequent release of preformed thyroid hormone. Laboratory studies reveal elevated thyroid hormones and decreased TSH. Unlike iodine 1 123 (123 I) or technetium TC 99m (99m Tc) radionuclide scans in patients with Graves disease, radionuclide scans in patients with subacute thyroiditis show decreased uptake by the thyroid gland. Once the inflammation resolves, thyroid-related symptoms resolve.
Because antithyroid medications do not prevent the release of preformed thyroid hormones, these agents are not useful.
Cardiac symptoms can be alleviated with propranolol. Anti-inflammatory medications, such as aspirin and corticosteroids, offer symptomatic relief.
Chronic lymphocytic thyroiditis
Like Graves disease, chronic lymphocytic (ie, Hashimoto) thyroiditis is an autoimmune disorder. However, in patients with chronic lymphocytic thyroiditis, antithyroglobulin and antithyroid peroxidase antibodies predominate. If present, TSI titers are low.
The hyperthyroid phase of chronic lymphocytic thyroiditis (hashitoxicosis) is self-limited and responds to antithyroid therapy. Antithyroid T lymphocytes and antibodies cause destruction of thyroid follicular cells, and hypothyroidism occurs over time.
The duration of the hyperthyroid phase of Hashimoto thyroiditis may be as long as 6 months.
One study found that 11.5% of patients with Hashimoto thyroiditis presented with hyperthyroidism. [5]
Epidemiology
Because Graves disease accounts for more than 95% of childhood cases of hyperthyroidism in the United States, the frequency of Graves disease approximates the frequency of all cases of hyperthyroidism. Prevalence of Graves disease is approximately 1 in 10,000 in the pediatric population [6] , accounting for fewer than 5% of the total US cases of Graves disease.
Graves disease is associated with human leukocyte antigen (HLA)-B8 and HLA-DR3 and is more common in some families than in others. Inheritance is polygenic. Monozygotic twins show 50% concordance for the disease, suggesting interplay between environmental and genetic factors.
Associations between Graves disease and other autoimmune diseases are well described and include associations with diabetes mellitus (DM), Addison disease, systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), myasthenia gravis, vitiligo, immune thrombocytopenic purpura (ITP), and pernicious anemia. Graves disease is more common in patients with trisomy 21 than in patients without trisomy 21.
One study estimated the incidence of hyperthyroidism in the United States in 2008 based on the number of new prescriptions of thionamides by age group and date from the 2008 US Census. [7] The study concluded that the incidence among individuals aged 0-11 years was 0.44 cases per 1000 population and that the incidence among individuals aged 12-17 years was 0.59 cases per 1000 population. [7] Thus, the incidence increases throughout childhood, with a peak incidence in children aged 10-15 years.
Sexual differences in incidence
Although females are affected by Graves disease more often than males, with a reported female-to-male ratio of 3-6:1, the frequency of neonatal Graves disease is equal in males and females.
Other causes of hyperthyroidism have no male or female preponderance. These include the hyperthyroidism of McCune-Albright syndrome, although the variant of this syndrome that includes precocious puberty is more common in girls than in boys.
Prognosis
The vast majority of pediatric patients with hyperthyroidism have an excellent prognosis. However, those diagnosed with Graves disease and treated in childhood may have a lower quality of life than thier healthy peers. [8] Signs of congestive heart failure (CHF) are rare in children. Ophthalmopathy of Graves disease is usually mild but may persist despite resolution of the hyperthyroidism.
Although the course of neonatal Graves disease is self-limited, the prognosis is considerably worse than that in older children. As a result of their disease, patients are prone to prematurity, airway obstruction, and heart failure. The mortality rate from these conditions has been as high as 16%. Even patients who are successfully treated may develop craniosynostosis and eventual developmental delay.
Complications from hyperthyroidism include the following:
-
Congestive heart failure (CHF)
-
Craniosynostosis (neonates)
-
Developmental delay (neonates)
-
Hypothyroidism
-
Hypercalcemia is occasionally seen in patients with hyperthyroidism.
Only one-third of pediatric patients with Graves disease achieve remission. In one study, the strongest predictor of remission was TRAb normalization timing. Patients who normalized TRAb levels in the first year had a 70% remission rate and among those who normalized in the second year of therapy, a 50% remission rate was seen. [2]
Complications
Graves orbitopathy (GO) is the main extrathyroidal manifestation of Graves disease, though severe forms are rare. In a study of 346 newly diagnosed patients at a single center over an 8-year period found moderate-to-severe and active GO or sight-threatening GO were rare at presentation and rarely develop during ATD treatment. Most patients (>80%) with no GO at baseline did not develop GO after an 18-month follow-up period. Remission of mild GO occurs in the majority of cases. [9]
In its 2016 guidelines, the European Thyroid Association/European Group on Graves Orbitopathy (EUGOGO) provides an algorithm for treatment selection based on activity and severity. [10]
Patient Education
Compliance
Patients who choose treatment with antithyroid medications should be educated on the importance of compliance, as noncompliance can be problematic. Patients with hyperthyroidism are prone to forgetting their medicine because of short attention span. Some patients skip their medicine as a way of controlling their weight.
Treatment adverse effects
Counsel patients regarding the common and uncommon adverse effects of treatment. At the first sign of a serious adverse effect, such as fever, rash, jaundice, arthritis, or mucocutaneous ulcer, their antithyroid medications should be discontinued; laboratory evaluation may be appropriate.
Patients and their parents should also be warned about excessive weight gain as the hyperthyroidism is corrected. Treatment of hyperthyroidism is associated with excessive weight gain, unless food intake is decreased.
Thyroid storm, thyrotoxicosis, hypothyroidism, and adrenergic agents
Patients with Graves disease and their parents should be also advised to take necessary precautions to avoid trauma to the neck area, as this could precipitate thyroid storm.
All patients monitored for hyperthyroidism should be aware of the signs and symptoms of thyrotoxicosis (should they have a relapse) and the signs and symptoms of hypothyroidism. Symptoms of hypothyroidism include fatigue, cold intolerance, hoarseness, constipation, muscle cramps, menstrual irregularities, and weight gain. Signs of hypothyroidism include dry skin, bradycardia, edema, and delayed relaxation of deep tendon reflexes.
In addition, because thyrotoxic symptoms largely mimic those of adrenergic excess, patients with hyperthyroidism should avoid taking any adrenergic agents. Advise patients not to take over-the-counter (OTC) cold remedies because many contain sympathomimetic agents (eg, pseudoephedrine) and can therefore exacerbate thyrotoxic symptoms.
-
Pediatric Hyperthyroidism. Schematic representation of the negative/positive feedback system with respect to the hypothalamic-pituitary-thyroid axis. TRH = thyrotropin-releasing hormone; TSH = thyroid-stimulating hormone.









